关于细胞周期,细胞增殖和细胞死亡的全面的综述,这些基于细胞的实验结果是来邦网从340份正式出版物中调查获得的。
A comprehensive review of assays for the cell cycle, cell proliferation, and cell death, along with the results for cell-based assays from a Labome survey of 340 formal publications.
细胞周期是真核细胞复制和分裂的过程。细胞周期由两个特定、独特的阶段组成:(1)间期: 由G1期(Gap 1),S期(合成),和G2期(Gap 2)组成;(2)有丝分裂期:M期(有丝分裂)。在间期,细胞生长(G1 期),累积复制必需的的能量,复制细胞DNA(S 期),并准备分裂(G2 期) [1]. 在这时间点上,细胞进入M期,其被分成两个严格调控的阶段:有丝分裂和胞质分裂。在有丝分裂,亲本细胞的染色体被分到两个姐妹细胞。在胞质分裂,细胞质分裂发生,导致两个特异子细胞的形成。细胞周期的每个阶段都是被严格调控的,检查点的存在是为了检测潜在的DNA损伤,并允许在细胞分裂前修复。如果损伤不能修复,细胞则会凋亡。细胞也能可逆地停止分裂并暂时进入静止或衰老状态—G0期。第一检查点是在G1期的末端,决定细胞是进入S期并分裂,还是延迟分裂,还是进入G0期。第二检查点在G2期的末端,如果细胞具备所有必需原件,则触发有丝分裂。
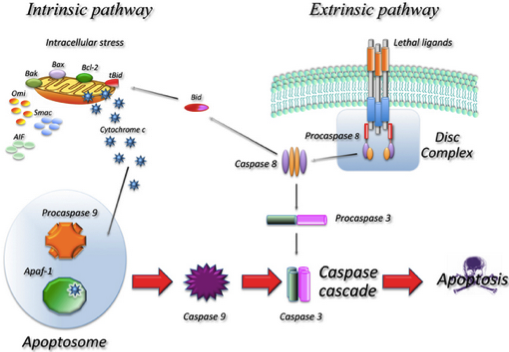
程序性细胞死亡在生物细胞和系统的维护中具有重要作用。虽然以下介绍的概述简略且简化,但是有几篇优秀的综述详细阐述了这些通路和通路的复杂性 [2, 3]. 程序性细胞死亡的“经典”方式,细胞凋亡,其特征是通过激活抑制子和效应物caspases,形成细胞皱缩,染色质压缩,膜出泡,线粒体完整性丧失和DNA片段化。它是一个严格调控和控制的主动过程,最终导致细胞分解,同时避免炎症反应。一旦分解,细胞碎片(凋亡小体)被运送到吞噬体。凋亡可以分成外源性(细胞表面受体的活化,例如肿瘤坏死因子α,其导致caspase -8的活化 [4] ) 和内源性,这可以通过caspase-9的激活来区分。细胞凋亡的许多方面在线粒体中被几个家庭蛋白调节,包括SMACs(小线粒体来源的caspases的激活子),IAPs(凋亡蛋白抑制物),以及Bcl-2家族,以及膜的极性和完整性。
坏死是与急性细胞应激相关的细胞死亡的一种方式,可以由毒素,感染或损伤引起。不像细胞凋亡,该过程是不可逆的,并且对细胞和机体总是有害的。细胞坏死期间,细胞表面受体的激活导致细胞质膜完整性丧失,凋亡蛋白不受控制地释放到细胞。这导致炎症反应广泛激活,阻止吞噬体去除死细胞,并导致坏死组织堆积。然而,细胞凋亡和坏死的成因和影响的区别是复杂的,有报道表明病原体导致的细胞死亡(称作坏死) [5, 6] 。
除了坏死和凋亡,程序性细胞死亡还有其他更特异的方式。失巢凋亡(Anoikis)是程序性细胞死亡的一种方式,其与细胞外基质的细胞分离有关。细胞焦亡(Pyroptosis)是一种促炎方式的细胞死亡,其与微生物感染有关,而且是唯一与caspase-1激活有关的细胞死亡方式 [7, 8] 。
细胞周期的几种方法在下面讨论。然而,必须记住,这些方法不是相互排斥的,并且对于最好的和最可靠的数据,多种染料和/或分析物可在一次实验或多个测定中进行组合使用。
有几种不同的染料可在这些实验中使用,包括碘化丙啶(PI),7-氨基放线菌素-D(7-AAD),Hoechst 33342和33258,以及4'6'二脒基-2-苯基吲哚(DAPI)。然而,大多数的FACS机器通常只包含单个氩离子激光器,这些需要紫外线活化的染料,如DAPI和Hoechst 33342不常使用。
进行这些分析时,重要的是要意识到,使用7-AAD或PI单染色的FACS分析,是无法区分非G1和G2期其分别来自非常早期的S期与非常晚期S期,同样情况也出现在G2和M期中。因此,有时需要这些染料结合增殖标记物如BrdU共同使用。这需要在研究初期增加一个额外的步骤,培养中的活细胞与BrdU孵育约30分钟,然后再与抗-BrdU和荧光耦联二抗孵育。细胞然后以上述方式进行测定。
进行细胞周期FACS数据分析时,有一些重要的因素要考虑。前向散射/侧向散射图是分析的不可分割的部分,不应被忽视,因为这表明有多少单细胞被识别。该分析中如果允许双峰(G1期两个细胞中的DNA含量被记录为单个的G2 / M事件),它可以导致G2 / M期的过度代表。细胞聚集和低于1000个细胞/秒的流速,也应避免使用低样品压力差,其导致形成方差系数(coefficient of variance,CV)。最后,含有正常二倍体DNA的参考样品应当包括附加控制。
The NNicoletti测定 [9] 是细胞周期的FACS分析的改良形式,凋亡可以由低完整的DNA含量,和高片段化DNA含量 [10] (前G1峰)同时检测测定。Nicoletti方法非常类似于上述方法,所不同的是用低渗缓冲液(如HFS缓冲液,其含有柠檬酸和triton X-100,或低渗荧光溶液)透化细胞。凋亡细胞染色弱,是由于细胞核酸酶的活化和低分子量DNA中扩散出细胞。固定和透化细胞可刺激寡聚核小体和单核小体的释放。使用低渗缓冲液可促进片段化的DNA的丢失,从而导致前G1峰的移位。
图4表明健康细胞(顶部),其一个细胞亚群开始经历凋亡(中部),并且有细胞在进行大量凋亡(底部)。然而,使用的Nicoletti测定时,必须小心从细胞碎片中区分凋亡细胞核,以确保在固定和染色过程中不会发生DNA剪切。
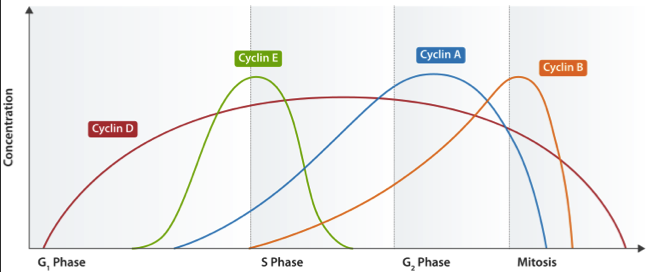
| 细胞周期蛋白 | 不同时期的表达峰谱 | Cdk 结合伴侣 |
|---|---|---|
| D | G1 | Cdk4, Ckd6 |
| E | G1/S | Cdk2 |
| A | S/G2 | Cdk1, Cdk2 |
| B | M | Cdk1 |
这些不同的表达模式可以在细胞周期分析中得以分析。总水平和/或单个细胞周期蛋白的磷酸化状态可以通过免疫印迹用特异性抗体简便且迅速地测量。此外,特定的ELISA试剂盒可用于测定单个细胞周期蛋白家族的成分,使得能够进行更大量的表达测定。最后,荧光耦联抗体可以在免疫组化或免疫细胞化学,或流式细胞术中使用。结合细胞周期蛋白染色和FACS方法,通过检测DNA含量,可以为准确分析细胞周期提供强大且定量的研究工具。
目前,有许多方法和试剂盒用于测量细胞增殖,线粒体功能的许多方面,也可以剪接地测量细胞活力。然而,实验的过度分析,信息的不正确解读,这些在已发表文献越来越频繁出现。在细胞增殖实验中,输出应该给你一个直接和准确的测量值,即细胞群体中积极分裂的细胞数,无论是培养中的细胞还是组织。与此相反,细胞活力测定实验是设计来指示细胞群体中“健康”细胞的数量,通常使用新陈代谢活跃细胞的特殊指示剂,经常与线粒体功能有关。与增殖实验不同,使用的测量值不会区分静止/衰老与积极分裂的细胞。
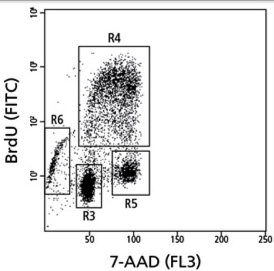
测定细胞增殖的传统方法是测定DNA合成,在细胞周期S期掺入标记的DNA类似物或前体(5-溴-2'-脱氧尿苷(BrdU)或[3H]–胸腺嘧啶,嘧啶的类似物,其能掺入到新合成DNA的胸腺嘧啶的地方)进入细胞基因组DNA。对于培养中的细胞,最常用的方法是通过比色ELISA法测定嵌入的BrdU(图6)。类似的方法可测定体内细胞的增殖,通过在收细胞前用BrdU脉冲标记组织,随后通过ELISA或免疫组织化学染色测量BrdU。这也可以通过流式细胞术测量。一个关于BrdU VS 7-AAD的例子见图7。
增殖细胞核抗原(PCNA)是细胞增殖另一种常用标记物。它与DNA聚合酶δ协同作用提高DNA合成速率,其是通过围绕基因组将复制酶固定到DNA来促进复制。DNA合成过程中,其在细胞核中表达,因此可被用作细胞增殖的标记物。它还在DNA修复中具有重要作用。
直接或间接地测量DNA合成的实验,本质上是细胞周期的敏感阶段。根据实验结果,有可能需要同步化细胞,无论是通过血清剥夺使其积累在G1期(这也影响细胞活力)还是化学抑制DNA合成阻断在S期,都需要胸腺嘧啶,阿糖胞苷胞嘧啶,羟基脲或氨基蝶呤。
现在有许多不同的可用代谢分析实验,下面讨论会有讨论。这些实验中,要么是测量重要的代谢物的量如ATP,要么是利用四氮唑盐(tetrazolium salts)或者刃天青染料(resazurin dyes)的减少。在细胞增殖时,NADPH/ NADP,FADH/ FAD,FMNH/ FMN和NADH/ NAD的比值会增大。在这些细胞脱氢酶或还原酶的代谢中间物的存在下,四氮唑盐被还原成甲臜(formazan)产物,其可以通过比色变化来检测。同样地,刃天青(7-羟基-3H-吩恶嗪-3-酮-10-氧化物)这种非荧光蓝色氧化还原染料被还原成异吩恶唑酮(resorufin),一种红色荧光化合物,其荧光和比色都有变化。下面介绍常用的底物,和他们的一些优点和缺点:
- MTT:MTT(3-(4,5-二甲基吡啶-2-基)-2,5-二苯基溴化四唑)是一种四氮唑盐,可被线粒体和外线粒体脱氢酶还原成不可溶的蓝色甲臜晶体,这意味实验读取前需要一个溶解步骤 [15]. 此外,在此实验中,细胞变得没有活力,也就是说在相同的实验板上不能进行重复或互补实验。
- MTS / XTT:MTS(3-(4,5-二甲基-2-基)-5-(3-羧基甲氧基)-2-(4-磺苯基)-2H-四唑)和XTT(2,3-二(2-甲氧基-4-硝基-5-磺苯基)-2H-四唑-5-酰替苯胺)底物类似MTT。然而,有一个优点是,在中级电子受体吩嗪硫酸甲酯(PMS)存在时,这个反应可以再细胞内进行,从而提高其灵敏度。此外,被还原的甲臜产物是可溶的,且可被释放到培养基,这就不再需要MTT中额外溶解度步骤。然而,有报道表明,长时间孵育,细胞培养基的酚红,脂肪酸和血清白蛋白可使MTS,XTT和WST实验存在失真的数据 [16].
- Alamar Blue:在活细胞中,刃天青化合物Alamar Blue被还原成异吩恶唑酮和二氢异吩恶唑酮。它可以在不裂解细胞的情况下进入活细胞,并稳定存在于培养基中。该方法具有可以使用荧光和比色板读数的优点。
- WST:水溶性四氮唑盐(Water soluble Tetrazolium Salts ,WSTs)是细胞不可渗透的四唑染料,可由细胞质膜电子传递在细胞外还原 [17], 并与电子受体PMS结合生成水溶性甲臜染料。
有许多有效的实验,其测量ATP水平来表征整体细胞健康程度。当细胞开始凋亡或失去膜的完整性,ATP酶分解ATP库,并阻止新的ATP的合成。这导致细胞内ATP水平的急剧下降。冷光ATP分析(如Promega’s CellTiter-Glo)通过裂解细胞释放ATP,而同时抑制ATPase的活力。在镁和ATP的存在下,荧光素酶氧化荧光素成氧化荧光素,使得冷光信号和细胞内ATP浓度直接相关的发光信号。 [18, 19].
当选择所需适当的代谢分析时,需要考虑许多因素。以上列出的每一个底物,或者本文没有涉及到的,都具有其独特的优点和缺点。检测的灵敏度,信噪比,易用性,和试剂的稳定性都是要考虑的因素。代谢分析的另一个重要注意事项是,与细胞内代谢活性的改变紧密相关的这些底物的减少,与整体细胞的活力没有直接的影响。因此,你想要回答的问题将在选择合适的实验中发挥关键的作用。
此类所有活力实验依赖于,当细胞活力丧失时细胞膜破裂,致使大分子进入细胞,或者使细胞内蛋白质分泌到培养基中。
- LDH :乳酸脱氢酶是普遍存在,稳定的胞质酶,将乳酸转化为丙酮酸。如果细胞膜被破坏, LDH从细胞中释放,可以在细胞培养基中检测其酶活。在乳酸盐转化为丙酮酸期间,NAD +被还原成NADH/ H +。基于LDH的活力实验是利用形成自由的氢离子,将NADH/ H +的H +转移到四氮唑盐INT(2-(4-碘苯基)-3-(4-硝基苯基)-5- p苯基四唑氯化物),还原成红色甲瓒染料 [20].
- 台盼蓝(Trypan Blue):悬浮细胞台盼蓝染色是最古老和最简单的细胞活力测定方法。健康活细胞的完整细胞膜阻止台盼蓝进入细胞。在死亡或濒临死亡的细胞,台盼蓝可进入细胞,将其染成蓝色。该方法传统定量是手动使用显微镜和血球计数器,这非常耗费人力。然而,自动细胞计数的使用使得实验耗时更少,比以前更准确。
- 钙黄绿素-AM:钙黄绿素-乙酰氧基甲基酯是一种非荧光染料,可用于细胞活力和细胞凋亡测定。它是亲脂性,从而可以通过细胞膜。一旦进入细胞,细胞内的酯酶裂解乙酰氧基基团的酯键,产生荧光阴离子且亲水的钙黄绿素染料,其能被截留在细胞内。无活性细胞不含有活性酯酶,这使得该实验可被用细胞活力的测定。在生理pH条件下,Cu2 +,Co2 +,Fe3 +,Mn2+和Ni2 +可以淬灭钙黄绿素的荧光信号,这意味着必须小心选择适当的细胞培养基。
- 碘化丙啶/7-AAD:如上所述,这些嵌入剂经常用于研究细胞周期。然而,由于它们不可透膜性,它们被活细胞截留在外。这意味着,荧光信号可以通过PI 进入无活性细胞,其可以通过荧光显微镜或流式细胞仪分析来分析。
评估细胞死亡已成为生物研究必不可少的一个组成部分,新技术以很快的速度在更新。虽然判断细胞死亡经典形式是细胞凋亡,也有额外的细胞死亡途径需要考虑,包括坏死,失巢凋亡和细胞焦亡。因此,你试图回答的以下问题,疾病,组织,细胞系或细胞死亡的诱因,都是最终实验设计的重要组成部分。然而,在即将死亡细胞中发生的过程是极其复杂的,涉及死亡途径交叉,所以区分不同途径经常不是直接的。
因为细胞凋亡是程序性细胞死亡途径,它是由许多有序,连续的步骤严格调控。例如,起始子caspase-8或-9的激活需要先激活效应子caspases-3 和 -7,这过程先于DNA断裂。这意味着你实验中的时间是至关重要的,并且通常需要许多时间点的测试,以有意义的方式观测合适的改变。总体而言,下文讨论的凋亡性细胞死亡的许多方面都可以评估。
凋亡的特征在于一些特定的细胞变化,包括细胞核和细胞质浓缩和碎裂,质膜出泡和形成凋亡小体。虽然不能准确量化的,这些变化可以通过光学显微镜在基础水平下显微可视(图8),并可用电子显微镜或时间推移显微镜在一个更基础的水平下可视。
Caspase的激活或剪切是细胞培养中评估细胞凋亡最常用的一种方法。凋亡途径的Caspase基于其结构和在细胞死亡过程的作用,可以分成两个组。具体来说,Caspase起始子在其前端功能域具有CARD(caspase募集结构域)或DED(死亡效应域;图9),而Caspase效应子只有很短的前端功能域。Caspase起始子(Caspase-2,-8,-9,和-10)负责起始特定Caspase级联反应。正因为如此,这些蛋白可以用于区分不同的细胞凋亡途径:Caspase -8是由胞外死亡受体激活,而Caspase -9参与胞内线粒体凋亡通路。Caspase效应子的活化(Caspase -3,-6和-7)通过在特定天冬氨酸残基剪切而激活通路,最终导致细胞破裂。Caspase活化的方法在图10中概述。
现在有多种方法来评估Caspase的活化,从向细胞加入荧光,比色和发光底物层面,到已剪切Caspase的蛋白质印迹和ELISA层面,后者可用于细胞提取物和固定的细胞,或者组织的免疫化学或免疫组织化学层面。Caspase底物测定法简单,通常使用微孔板。将含荧光前体,比色前体或发光前体的细胞裂解和活性混合缓冲液直接加入细胞培养的培养基。样品中活性Caspase然后剪切底物,形成能在特定波长被激发的荧光或发光产物。对于此处所讨论的关键Caspase底物的实例在表2。
| 结构序列 | 目标 Caspase | 剪切产物 | 输出方式 |
|---|---|---|---|
| VDVAD-AFC | 2 | AFC | 荧光 |
| DEVD-AMC | 3, 7 | AMC | 荧光 |
| Z-LEHD-R110 | 9 | R110 | 荧光和比色 |
| AC-IETD-AFC | 8 | AFC | 荧光 |
为提高这些实验的可靠性,几个特异的Caspase抑制剂可作为对照,以证明各自底物的特异性。例如, AC-DEVD-CHO(Caspase-3/7抑制剂),AC-DEVD-FMK(pan Caspase抑制剂),Z-IETD-FMK(Caspase-8抑制剂)和Z-LEHD-FMK(Caspase9抑制剂)。这些试剂形成了有力的手段来评估凋亡,虽然必须考虑更适合您的需求的底物。
除了设备的明显实用性,还有一些重要的因素包括灵敏度和所用底物的输出。比色底物形成有色的产物,在可见光范围内吸收光。从实验得到的信号正比于被测量的分析物。这些实验干净的板子就可进行,底物一般都更稳定,比荧光或发光底物更划算。荧光底物产生反应产物,其荧光可被特定波长光激活,且检测出的RFU(相对荧光单位)正比于被测量的分析物。这些底物更易淬灭,实验条件和物理性质的信号干扰,例如指纹,略微比色测定法更为敏感。然而,它们显著扩大实验动态范围,可以有非常高的读数,因为它们不受比色测定中OD 2-4的限制。荧光测定法需要黑色孔板,防止荧光信号浸出。发光的Caspase底物是基于生物发光化合物,如荧火虫荧光素酶。发光是荧光素酶的光线发射,此时电子从激发态回到基底态。这些反应需要白壁孔板,并且通常比荧光底物灵敏好几倍,具有较低背景信号,以及检测方法更快。然而有些,像荧光底物,是非常昂贵的。除了检测活力的实验,主要Caspase(-3/7,-8,-9)的高品质抗体的也可利用。这些抗体能够在蛋白质印记,免疫细胞化学和免疫组化试验中,选择性检测Caspase其全长(无活性),全长和片段,或仅片段(有活性)。
细胞染色体的崩解是细胞凋亡的关键步骤,有许多方法将其量化。从历史上看,DNA片段由简单的凝胶电泳检测,在某些领域今天仍在使用。虽然这种方法显示了DNA崩解,但不容易量化。反而,有几个ELISA试剂盒可用于测量DNA的碎片。这些试剂盒可以以夹心ELISA形式检测从受损细胞释放的BrdU标记的片段,给出可量化和统计分析的数据。同样,试剂盒可用于特异性检测细胞质中组蛋白相关的DNA片段,使用的是组蛋白捕获抗体将核小体捕获到微孔板,随后使用DNA二级抗体。
其他检测DNA片段的方法,是利用能将标记核苷酸添加到DNA片段末端的酶。使用这种技术的传统方法是TUNEL(末端UTP缺口末端标记),它使用末端脱氧核苷酸转移酶(TdT)添加荧光或比色标记物到DNA的黏性或平头末端。TUNEL法是经常用于检测组织切片中的细胞凋亡,但也可用在细胞培养的免疫细胞化学,流式细胞术或原位荧光检测中。
Bcl-2家族蛋白是线粒体介导凋亡的重要调节剂。该家族包括促凋亡和抗凋亡蛋白,协同调节线粒体外膜的电势(关于这个问题有几篇全面的综述 [21-23] ). 虽然Bcl-2家族蛋白是重要的凋亡调节剂,但是它们的绝对水平并不是凋亡指示剂。相反,它们磷酸化状态和/或构象的变化是很重要的。可以测定的主要成员和变化在几篇优秀的综述中得以概括 [22, 24].这些变化可以通过多种方法测定,包括基本的免疫印迹,ELISA或蛋白的构象免疫沉淀如Bax蛋白。 为了检测Bax蛋白的活化,测定实验利用当Bax被激活准备插入到线粒体膜时发生构象变化。这个构象变化暴露Bax的N-20表位,该蛋白质N末端20个氨基酸区域。如果Bax蛋白在基于CHAPS的温和缓冲液中被免疫沉淀,该蛋白质的构象保持不变。这可因此被利用来确定总活性蛋白质的比率。
凋亡也伴随线粒体膜电位(Δψm)的改变,它可以由Bcl-2家族成员形成的气孔,或内膜通透性孔复合(PTPC)的开口而引起。这可以通过使用荧光探针在活细胞中进行测定。染料包括若丹明123,TMRE,和JC-1。这些染料通常是亲脂和阳离子的,这意味着线粒体具有更高的负Δψm(极化;健康)的将比去极化的不健康线粒体积累更多的染料 [25]. 为确保数据的正确性,荧光染料必须被最优化利用,且染料的选择取决于所问的问题。例如,JC-1是常用于测定Δψm导致的细胞凋亡。当JC-1在线粒体中积累,其荧光发生从绿色(〜529纳米)到红色(〜590纳米)的改变。这使得线粒体去极化通过红色/绿色荧光强度比的减少来测定,着可以通过荧光显微镜或流式细胞仪来检测。TRMR / TMRE和若丹明123通常分别用于慢速和快速地解决紧急研究 [26], 并且通常通过流式细胞术检测,其使用共孵育线粒体特异性探针例如若丹明123或MitoTracker。
细胞色素c是电子传递链的基本组成部分,并且被发现与线粒体内膜相关且存在健康细胞的膜间隙。在细胞凋亡的起始阶段,细胞色素C被释放到细胞质中,在那里它结合Apaf-1(凋亡蛋白酶活化因子1),从而导致Caspase-3的剪切和活化。这样,检测细胞色素c的释放是测定早期凋亡的有力工具。有许多不同的方式来检测这一点,这些方法在灵敏度,准确度和所花费的时间上各有不同。首先,细胞培养可以分离细胞质和线粒体区室,且细胞色素C可以通过免疫印迹进行测定,并且同一抗体也可用于免疫细胞化学。这两种技术是劳动密集型的,并且当进行细胞分级时必须小心使用合适的对照,确保没有其他细胞区室的污染。也可以在洋地黄皂苷选择性透过质膜后,使用流式细胞仪检测细胞色素c释放 [27] 。
细胞内钙离子在许多生命过程中起着关键的调节作用,包括细胞凋亡 [28]. 迅速升高的线粒体钙离子水平是促凋亡信号,其形成线粒体中肿胀,导致线粒体外膜破裂。这以细胞色素c和其它凋亡因子包括AIF和Smac / DIABLO释放到细胞质而告终。活细胞线粒体钙离子水平变化可以使用合成染料,例如若丹明-2或罗丹明-FF进行检测。然而,这些技术是具有挑战性的,因为这些染料可渗出线粒体,随着时间失去效力,并且本身可以破坏线粒体形态 [29]. 因此,最准确检测线粒体钙离子水平的方法是使用特异性靶向线粒体的荧光指示剂,并结合使用荧光显微镜。关于这些染料有几个例子,包括放射性成像仪 [30]. 虽然由于实验的复杂性,关于线粒体代谢和信号研究,使用线粒体钙离子水平作为凋亡特异性指示剂仍然不是很理想,但是还是有许多更直接的方法检测细胞凋亡。
有几种方法可用来检测细胞质膜完整性的改变。磷脂酰丝氨酸(PS)是一种带负电荷的膜磷脂,通常专门在质膜内叶存在。在细胞凋亡早期阶段,膜的电荷比被打乱, PS暴露于细胞表面 [31]. 这可以通过使用膜联蛋白-V,磷脂和钙结合蛋白,偶联FITC或替代荧光染料,用流式细胞术来检测 [32], 这些测定通常与结合碘化丙啶(或7-氨基放线菌素D)同时来检测膜完整性。这可显示健康(膜联蛋白V和PI阴性;图11左下象限),早期凋亡(膜联蛋白V阳性,PI阴性;图11是左上象限),坏死(膜联蛋白V阴性,PI阳性;图11右下象限),或死亡(膜联蛋白V和PI阳性;图11右上象限)细胞之间的区别。
作为细胞凋亡过程的一部分,有另外的细胞蛋白被分解,其可以用作细胞凋亡标记物。其中的最好的例子是PARP剪切。 PARP(聚ADP核糖聚合酶)是至少有七个核蛋白的家族。在单链DNA断裂,PARP被激活,进行构象变化,并结合到受损区域,招募修复机器 [33]. 凋亡期间,活化的caspase-3剪切PARP,阻止DNA修复。PARP被剪切的片段可以很容易通过蛋白质印记来检测,其被用作细胞凋亡的指示剂。
如上述讨论,细胞凋亡和坏死在细胞内稳态和死亡中具有非常不同的作用。所采用实验的最简单区别是炎症反应的诱导,虽然必须要指出的是,这些复杂的过程可能并不总是符合简单的传统的范例。在这最简单的定义中,术语“坏死”仅仅指的是一个大量的死细胞,并且是独立于细胞死亡通路 [3]. 虽然有些实验,例如台盼蓝可用于证明膜破裂,但是这些实验不能提供导致细胞死亡相关途径信息。因此,测定坏死的最佳方法是利用细胞死亡后发生的形态变化,并使用光学或电子显微镜来检测(图8)。具体来说,坏死的细胞表现出细胞核肿胀,染色质凝聚,核嗜碱性丢失,胞质结构和细胞器的功能崩解,肿胀而细胞溶解并导致细胞膜破裂。不过说实话,凋亡细胞也有与坏死一致的细胞膜破裂,这叫作凋亡坏死。发生这种情况时,当无吞噬作用时凋亡小体已经形成,这可能会导致小体的破裂。因此,难以确定坏死细胞是否实际上已死亡。
失巢凋亡是细胞凋亡的一种形式,导致细胞从细胞外基质上脱落。检测该现象在癌症研究中特别有用,因为肿瘤细胞获得不依赖贴壁生长(导致在体内系统中转移)的能力,并成为耐失巢凋亡。有因此许多检测体系可用来测定失巢凋亡。在这些体系中,细胞生长在涂有某种基质的板上,例如聚HEME(聚(2-羟乙基甲基丙烯酸酯)),以防止细胞附着。细胞然后用两种荧光染料共染色。首先,细胞活力由钙黄绿素-AM监测,其在485nm激发光,515nm发射光下使用荧光显微镜或荧光板读数器测得。失巢凋亡的检测可以同时加入只能进入受损细胞的荧光DNA结合染料,例如乙啡啶二聚体(激发光525nm和发射光590nm)。这些染料与DNA结合后发出荧光,可以在板读取器上获得失巢凋亡定量分析,或用荧光显微镜定性分析。除了专门的试剂盒,只要细胞能悬浮生长并不附着在基质上,许多作者使用简单的细胞凋亡实验,如DNA断裂或剪切ELISA,来检测细胞凋亡 [34].
细胞焦亡是最近被鉴定的细胞死亡途径,由Caspase-1专一介导的细菌感染,也导致促炎症细胞因子的激活,包括IL-1和IL-18。尽管与坏死相像,细胞焦亡同时导致膜破裂和炎症标记物的释放,这条途径可以通过Caspase-1激活加以区分 [35]. 因为Caspase-1不参与其他形式的细胞死亡如细胞凋亡 [36], 通过免疫印迹或ELISA剪切的caspase-1,可以简单快速地检测分离的细胞裂解物中的细胞焦亡。
这部分由来邦网提供,以帮助研究人员确定最适合的细胞检测试剂盒,以确定最适合的基于细胞的测定法的试剂盒。 来邦网 从正式出版物中调查获得 。 表3列出的用于细胞检测的试剂/试剂盒的主要供应商,和来邦网统计的其在出版物被使用的次数。
| 试剂/试剂盒 | 数目 | 方法 | 主要供应商* 和出版物数量 |
|---|---|---|---|
| 碘化丙啶 | 35 | 细胞化学, 流式细胞术 | Sigma (22) [37-58], Invitrogen (9) [59-67] |
| Hoechst 33342 | 37 | 细胞化学, 流式细胞术 | Invitrogen (26) [47, 67-91], Sigma (10) [59, 92-100] |
| Hoechst 33258 | 12 | 细胞化学 | Sigma (7) [101-107], Invitrogen (4) [108-111] |
| 7-氨基-放线菌素D | 16 | 细胞化学, 流式细胞术 | Becton Dickinson (8) [99, 112-118], Invitrogen (4) [119-122], Calbiochem (2) [123, 124] |
| BrdU | 84 | 免疫化学, 流式细胞术 | Sigma (27) [50, 70, 77, 102, 125-147], BD (26) [46, 60, 104, 109, 117, 136, 140, 144, 148-165], Abcam (8) [72, 128, 135, 166-170], Roche (8) [72, 85, 139, 171-175], DAKO (4) [127, 176-178], Accurate (4) [73, 179-181] |
| 细胞增殖试剂盒 | 44 | 细胞化学, 流式细胞术 | Promega: CellTiter 96 AQueous (25) [120, 154, 182-204], Invitrogen (8): CellTrace (6) [74, 121, 205-208], Roche (8): WST-1 (3) [209-211] |
| 细胞活性试剂盒 | 11 | 细胞化学, 流式细胞术 | Promega: CellTiter-Glo (8) [48, 78, 201, 212-216] |
| 细胞凋亡检测试剂盒 | 62 | 细胞化学, 流式细胞术 | EMD Millipore (27): ApopTag (21) [62, 77, 130, 198, 217-233], Roche (9): TUNEL (9) [144, 234-241], BD Biosciences (7) [88, 163, 187, 197, 242-244], Premega (6) [245-250] |
- Fink S, Cookson B. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73:1907-16 pubmed
- Hay S, Kannourakis G. A time to kill: viral manipulation of the cell death program. J Gen Virol. 2002;83:1547-64 pubmed
- Moss J, Aliprantis A, Zychlinsky A. The regulation of apoptosis by microbial pathogens. Int Rev Cytol. 1999;187:203-59 pubmed
- Hilbi H, Moss J, Hersh D, Chen Y, Arondel J, Banerjee S, et al. Shigella-induced apoptosis is dependent on caspase-1 which binds to IpaB. J Biol Chem. 1998;273:32895-900 pubmed
- Chen Y, Smith M, Thirumalai K, Zychlinsky A. A bacterial invasin induces macrophage apoptosis by binding directly to ICE. EMBO J. 1996;15:3853-60 pubmed
- Nicoletti I, Migliorati G, Pagliacci M, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271-9 pubmed
- Riccardi C, Nicoletti I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat Protoc. 2006;1:1458-61 pubmed
- Gautier J, Minshull J, Lohka M, Glotzer M, Hunt T, Maller J. Cyclin is a component of maturation-promoting factor from Xenopus. Cell. 1990;60:487-94 pubmed
- Gautier J, Norbury C, Lohka M, Nurse P, Maller J. Purified maturation-promoting factor contains the product of a Xenopus homolog of the fission yeast cell cycle control gene cdc2+. Cell. 1988;54:433-9 pubmed
- Murray A. Recycling the cell cycle: cyclins revisited. Cell. 2004;116:221-34 pubmed
- Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311-22 pubmed
- Liu Y, Peterson D, Kimura H, Schubert D. Mechanism of cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction. J Neurochem. 1997;69:581-93 pubmed
- Huang K, Chen Y, Walker A. Inaccuracies in MTS assays: major distorting effects of medium, serum albumin, and fatty acids. Biotechniques. 2004;37:406, 408, 410-2 pubmed
- Berridge M, Herst P, Tan A. Tetrazolium dyes as tools in cell biology: new insights into their cellular reduction. Biotechnol Annu Rev. 2005;11:127-52 pubmed
- Lundin A, Hasenson M, Persson J, Pousette A. Estimation of biomass in growing cell lines by adenosine triphosphate assay. Methods Enzymol. 1986;133:27-42 pubmed
- Crouch S, Kozlowski R, Slater K, Fletcher J. The use of ATP bioluminescence as a measure of cell proliferation and cytotoxicity. J Immunol Methods. 1993;160:81-8 pubmed
- Decker T, Lohmann-Matthes M. A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. J Immunol Methods. 1988;115:61-9 pubmed
- Lemasters J, Ramshesh V. Imaging of mitochondrial polarization and depolarization with cationic fluorophores. Methods Cell Biol. 2007;80:283-95 pubmed
- Campos C, Paim B, Cosso R, Castilho R, Rottenberg H, Vercesi A. Method for monitoring of mitochondrial cytochrome c release during cell death: Immunodetection of cytochrome c by flow cytometry after selective permeabilization of the plasma membrane. Cytometry A. 2006;69:515-23 pubmed
- Nagai T, Sawano A, Park E, Miyawaki A. Circularly permuted green fluorescent proteins engineered to sense Ca2+. Proc Natl Acad Sci U S A. 2001;98:3197-202 pubmed
- Van Heerde W, de Groot P, Reutelingsperger C. The complexity of the phospholipid binding protein Annexin V. Thromb Haemost. 1995;73:172-9 pubmed
- McFall A, Ulku A, Lambert Q, Kusa A, Rogers-Graham K, Der C. Oncogenic Ras blocks anoikis by activation of a novel effector pathway independent of phosphatidylinositol 3-kinase. Mol Cell Biol. 2001;21:5488-99 pubmed
- Hilbi H, Chen Y, Thirumalai K, Zychlinsky A. The interleukin 1beta-converting enzyme, caspase 1, is activated during Shigella flexneri-induced apoptosis in human monocyte-derived macrophages. Infect Immun. 1997;65:5165-70 pubmed
- Li P, Allen H, Banerjee S, Franklin S, Herzog L, Johnston C, et al. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell. 1995;80:401-11 pubmed
- Yang Y, Zhao Y, Liao W, Yang J, Wu L, Zheng Z, et al. Acetylation of FoxO1 activates Bim expression to induce apoptosis in response to histone deacetylase inhibitor depsipeptide treatment. Neoplasia. 2009;11:313-24 pubmed
- Leemput J, Masson C, Bigot K, Errachid A, Dansault A, Provost A, et al. ATM localization and gene expression in the adult mouse eye. Mol Vis. 2009;15:393-416 pubmed
- Le Clorennec C, Ouk T, Youlyouz-Marfak I, Panteix S, Martin C, Rastelli J, et al. Molecular basis of cytotoxicity of Epstein-Barr virus (EBV) latent membrane protein 1 (LMP1) in EBV latency III B cells: LMP1 induces type II ligand-independent autoactivation of CD95/Fas with caspase 8-mediated apoptosis. J Virol. 2008;82:6721-33 pubmed publisher
- Langlois S, Cowan K, Shao Q, Cowan B, Laird D. Caveolin-1 and -2 interact with connexin43 and regulate gap junctional intercellular communication in keratinocytes. Mol Biol Cell. 2008;19:912-28 pubmed
- Naderi A, Hughes-Davies L. A functionally significant cross-talk between androgen receptor and ErbB2 pathways in estrogen receptor negative breast cancer. Neoplasia. 2008;10:542-8 pubmed
- Kim S, Hur W, Choi J, Kim D, Wang J, Yoon H, et al. Functional characterization of human oncoprotein gankyrin in Zebrafish. Exp Mol Med. 2009;41:8-16 pubmed
- Scorah J, McGowan C. Claspin and Chk1 regulate replication fork stability by different mechanisms. Cell Cycle. 2009;8:1036-43 pubmed
- Peng Q, Masuda N, Jiang M, Li Q, Zhao M, Ross C, et al. The antidepressant sertraline improves the phenotype, promotes neurogenesis and increases BDNF levels in the R6/2 Huntington's disease mouse model. Exp Neurol. 2008;210:154-63 pubmed
- Chai Z, Sarcevic B, Mawson A, Toh B. SET-related cell division autoantigen-1 (CDA1) arrests cell growth. J Biol Chem. 2001;276:33665-74 pubmed
- Li S, Mo Z, Yang X, Price S, Shen M, Xiang M. Foxn4 controls the genesis of amacrine and horizontal cells by retinal progenitors. Neuron. 2004;43:795-807 pubmed
- Rhee H, Polak L, Fuchs E. Lhx2 maintains stem cell character in hair follicles. Science. 2006;312:1946-9 pubmed
- Lee J, Byun D, Lee M, Ryu B, Kang M, Chae K, et al. Frequent epigenetic inactivation of hSRBC in gastric cancer and its implication in attenuated p53 response to stresses. Int J Cancer. 2008;122:1573-84 pubmed
- Mailleux A, Overholtzer M, Schmelzle T, Bouillet P, Strasser A, Brugge J. BIM regulates apoptosis during mammary ductal morphogenesis, and its absence reveals alternative cell death mechanisms. Dev Cell. 2007;12:221-34 pubmed
- Won S, Kim S, Xie L, Wang Y, Mao X, Jin K, et al. Reelin-deficient mice show impaired neurogenesis and increased stroke size. Exp Neurol. 2006;198:250-9 pubmed
- Tang L, Alger H, Pereira F. COUP-TFI controls Notch regulation of hair cell and support cell differentiation. Development. 2006;133:3683-93 pubmed
- Li Y, Takemura G, Kosai K, Yuge K, Nagano S, Esaki M, et al. Postinfarction treatment with an adenoviral vector expressing hepatocyte growth factor relieves chronic left ventricular remodeling and dysfunction in mice. Circulation. 2003;107:2499-506 pubmed
- Wheeler M, Smutney O, Check J, Rusyn I, Schulte-Hermann R, Thurman R. Impaired Ras membrane association and activation in PPARalpha knockout mice after partial hepatectomy. Am J Physiol Gastrointest Liver Physiol. 2003;284:G302-12 pubmed
- Gastaldello S, D'Angelo S, Franzoso S, Fanin M, Angelini C, Betto R, et al. Inhibition of proteasome activity promotes the correct localization of disease-causing alpha-sarcoglycan mutants in HEK-293 cells constitutively expressing beta-, gamma-, and delta-sarcoglycan. Am J Pathol. 2008;173:170-81 pubmed publisher
- Simon D, Vadakkadath Meethal S, Wilson A, Gallego M, Weinecke S, Bruce E, et al. Activin receptor signaling regulates prostatic epithelial cell adhesion and viability. Neoplasia. 2009;11:365-76 pubmed
- Yap O, Bhat G, Liu L, Tollefsbol T. Epigenetic modifications of the Estrogen receptor beta gene in epithelial ovarian cancer cells. Anticancer Res. 2009;29:139-44 pubmed
- Seder C, Hartojo W, Lin L, Silvers A, Wang Z, Thomas D, et al. Upregulated INHBA expression may promote cell proliferation and is associated with poor survival in lung adenocarcinoma. Neoplasia. 2009;11:388-96 pubmed
- Drel V, Pacher P, Ali T, Shin J, Julius U, el-Remessy A, et al. Aldose reductase inhibitor fidarestat counteracts diabetes-associated cataract formation, retinal oxidative-nitrosative stress, glial activation, and apoptosis. Int J Mol Med. 2008;21:667-76 pubmed
- Nishitsuji K, Tomiyama T, Ishibashi K, Ito K, Teraoka R, Lambert M, et al. The E693Delta mutation in amyloid precursor protein increases intracellular accumulation of amyloid beta oligomers and causes endoplasmic reticulum stress-induced apoptosis in cultured cells. Am J Pathol. 2009;174:957-69 pubmed publisher
- Fan H, Zhao Z, Cheng J, Su X, Wu Q, Shan Y. Overexpression of DNA methyltransferase 1 and its biological significance in primary hepatocellular carcinoma. World J Gastroenterol. 2009;15:2020-6 pubmed
- 来邦网
- 英文来邦
- 试剂